Can’t-Miss Takeaways Of Tips About How To Get Rid Of Primer Dimer

The easiest is probably to increase your annealing temperature either for the earliest cycles of your pcr reaction (using a touchdown protocol), or for all cycles.
How to get rid of primer dimer. So your cycling conditions would be like 95 2. For that i did touch down pcr starting from 5 deg above tm and going down to 2 deg below tm. As g and c are strong matches,.
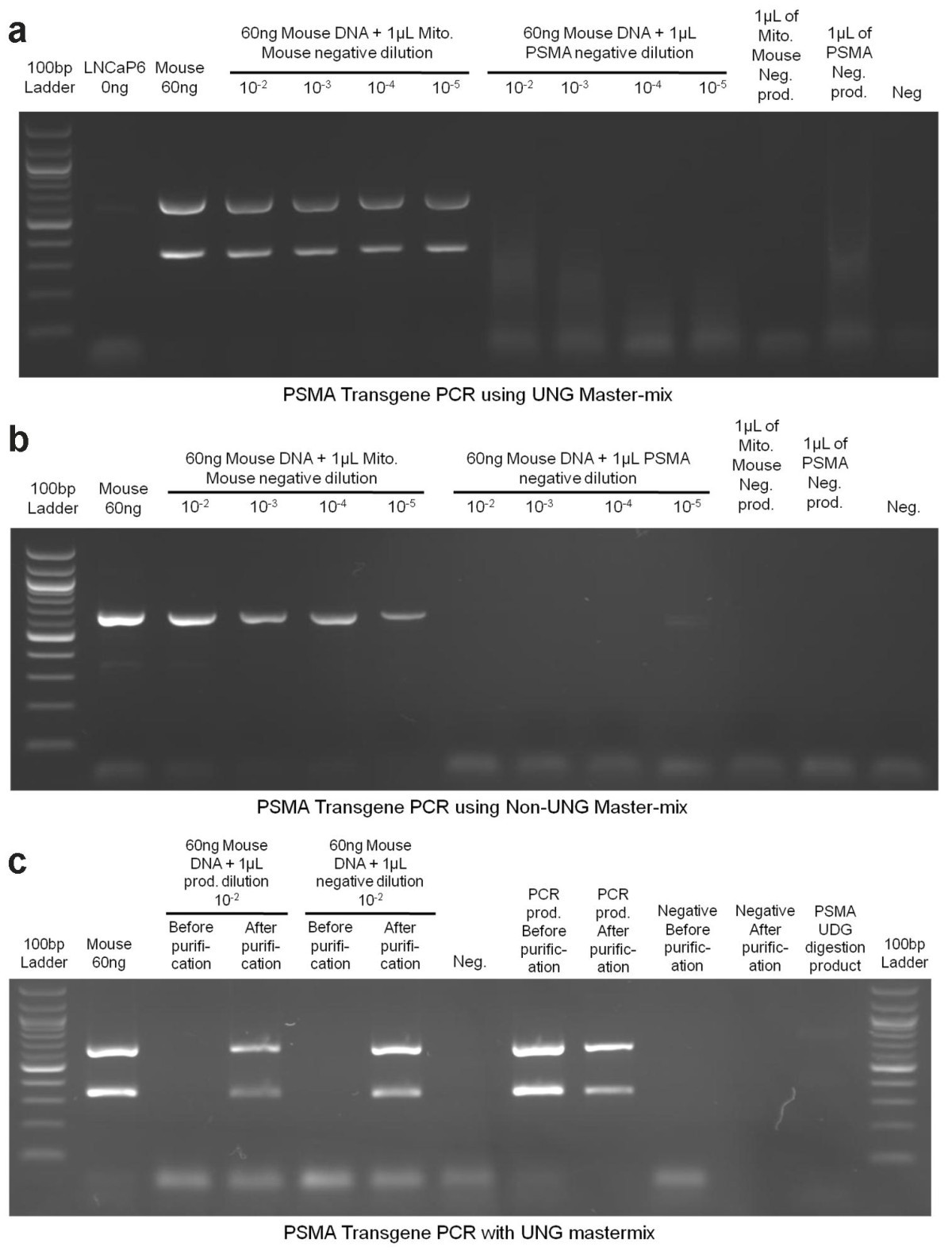
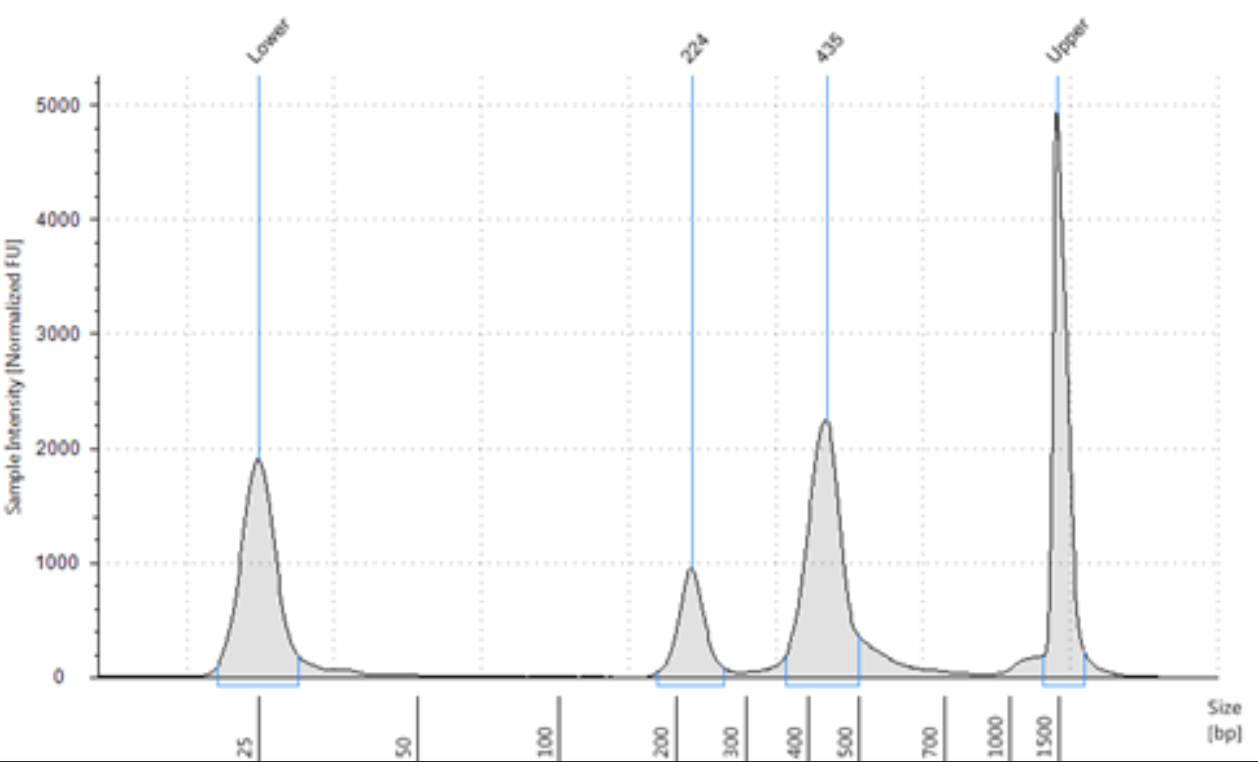
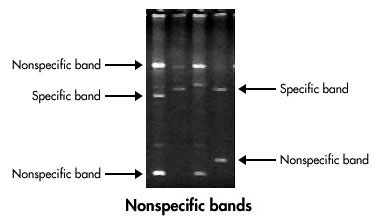
Bouttela, 50 bp bands are your primer dimers. Now that the damage is done, you might. Primer dimer and amplicon) well enough.
You can first run the product on agarose gel and separate the two bands (i.e. All info are in the quickchange manual. With this exovii) at 37c for 20 minutes using 1 ul enzyme and the accompanying.
If you make dilutions of your primers, would be beneficial to use te instead of h2o. Use a good primer design software. Problem with primer dimer can be overcome optimising lower concentration of magnesium chloride, using higer concen of templates and dmso 2 to not more than10%.
2) reduced amount of initial template used in pcr (from 50ng to 25ng) 3) added 2% dmso to the mastermix. I believe primer dimers are result of degradation of your primers. Then you can excise the amplicon bands from the gel.
1) reduced primer concentration from 0.5um to 0.25um. Set up the reaction on ice, this will not let the taq keep adding dntps to primer secondary structures 2. Strand doubling happens in the special cells that they supply with the kit.